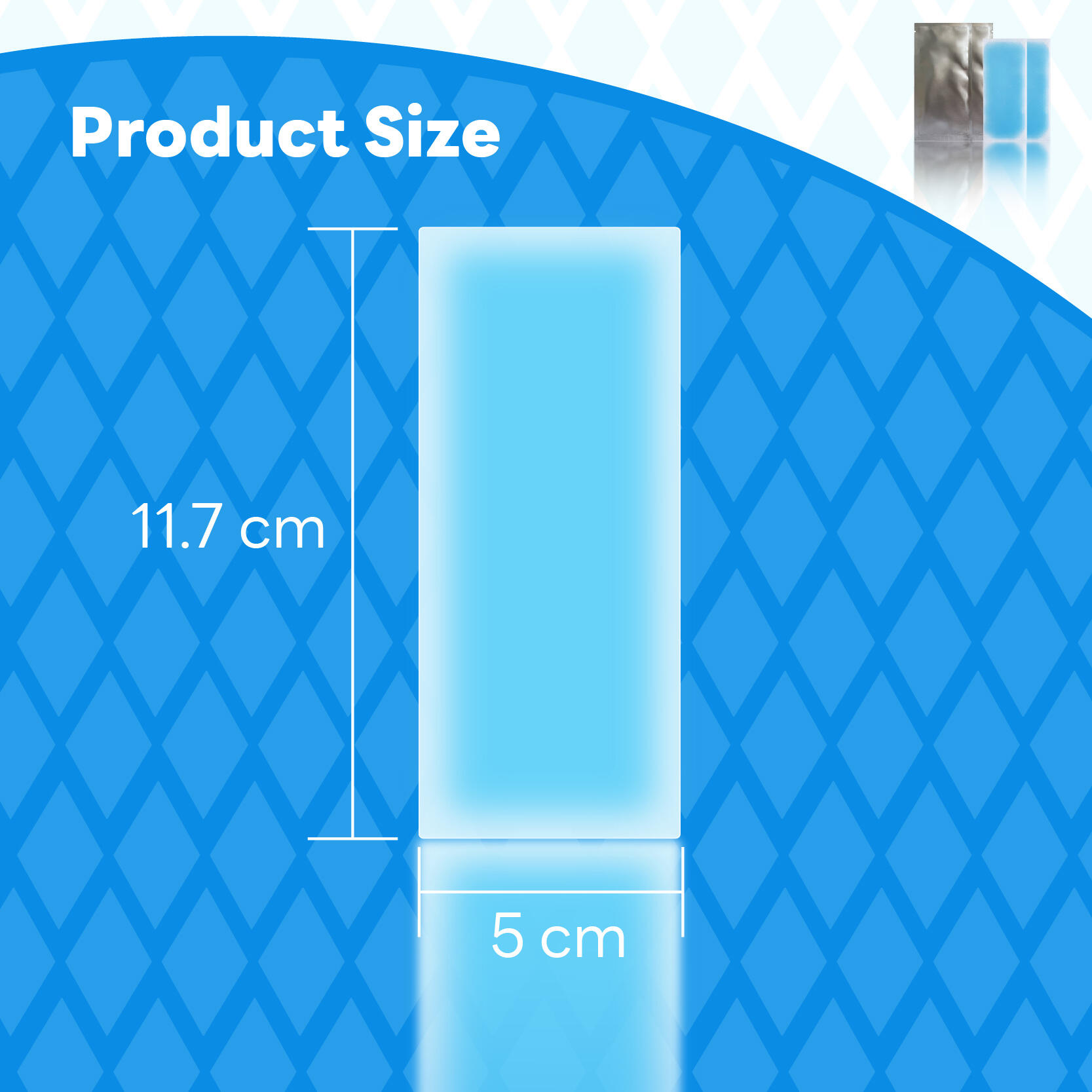
Modern pain management has evolved significantly with the development of topical solutions that deliver targeted relief directly to affected areas. Pain relief patches represent a revolutionary approach to managing discomfort, offering localized treatment without the systemic effects often associated with oral medications. These innovative medical devices combine multiple active ingredients with advanced delivery systems to provide sustained relief for various types of pain, from muscle aches to joint discomfort.
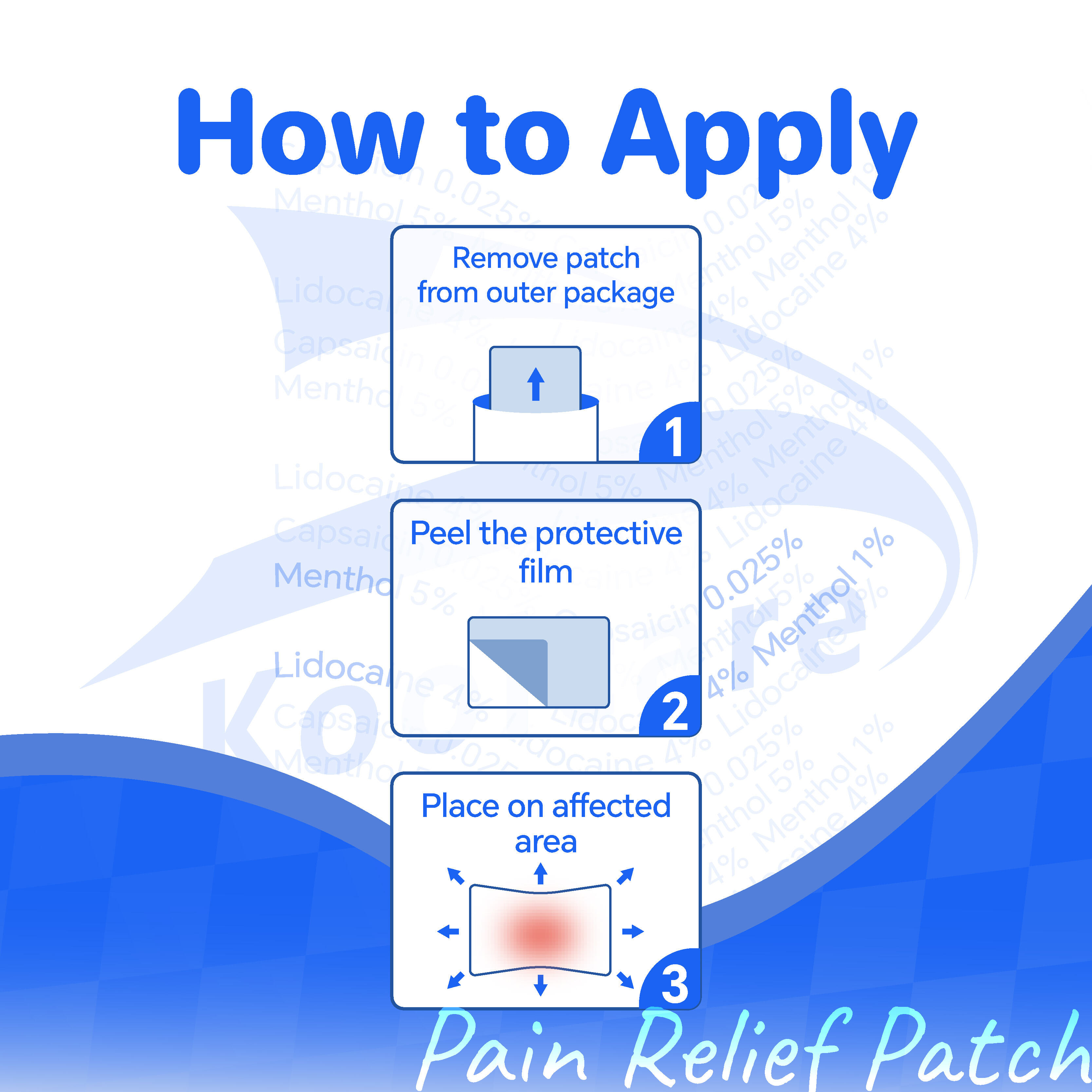
The effectiveness of these topical applications lies in their carefully formulated composition, which typically includes both active pharmaceutical ingredients and supporting compounds that enhance absorption and extend therapeutic duration. Understanding the components found in these patches helps consumers make informed decisions about their pain management options and ensures they select products that align with their specific needs and medical conditions.
Healthcare professionals increasingly recommend transdermal patches as part of comprehensive pain management strategies, particularly for patients seeking alternatives to traditional oral analgesics. The controlled release mechanism of these patches provides consistent medication delivery over extended periods, often lasting between four to twelve hours depending on the formulation and manufacturer specifications.
Active Pharmaceutical Ingredients in Topical Pain Management
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
The most commonly utilized active ingredients in topical pain management patches belong to the NSAID family, which includes diclofenac, ibuprofen, and ketoprofen. These compounds work by inhibiting cyclooxygenase enzymes, thereby reducing the production of prostaglandins that contribute to inflammation and pain sensation. Diclofenac, in particular, has demonstrated exceptional efficacy in transdermal applications due to its favorable skin penetration properties and sustained therapeutic effect.
Clinical studies have consistently shown that topical NSAIDs provide comparable pain relief to their oral counterparts while minimizing gastrointestinal side effects. The localized application allows for higher concentrations of the active ingredient to reach the target tissue while maintaining lower systemic exposure. This targeted approach makes NSAID-based patches particularly suitable for treating localized musculoskeletal conditions such as osteoarthritis, sports injuries, and repetitive strain injuries.
The formulation of NSAID patches requires careful consideration of drug concentration, release kinetics, and skin compatibility. Manufacturers often incorporate penetration enhancers and stabilizing agents to optimize drug delivery and ensure consistent therapeutic outcomes. The pH balance and adhesive properties must be precisely calibrated to maintain skin integrity while facilitating optimal drug absorption throughout the wear period.
Topical Analgesics and Counterirritants
Counterirritant compounds such as menthol, camphor, and methyl salicylate represent another significant category of active ingredients found in pain relief formulations. These substances create cooling or warming sensations that help mask pain signals through the gate control theory mechanism. Menthol, derived from peppermint oil, activates cold receptors in the skin, producing a cooling effect that can temporarily override pain perception.
Capsaicin, extracted from chili peppers, functions through a different mechanism by depleting substance P from nerve endings, ultimately reducing the transmission of pain signals to the brain. This ingredient requires careful handling during manufacturing and may cause initial burning sensations upon application, though this typically subsides with continued use. The concentration of capsaicin in patches varies depending on the intended application, with higher concentrations reserved for chronic pain conditions under medical supervision.
The combination of multiple counterirritants in a single patch formulation can provide synergistic effects, offering both immediate symptomatic relief and longer-term therapeutic benefits. However, the selection and concentration of these ingredients must be carefully balanced to avoid skin irritation while maintaining therapeutic efficacy throughout the designated wear period.
Supporting Components and Delivery Enhancement Systems
Penetration Enhancers and Absorption Promoters
The effectiveness of pain relief patches depends significantly on their ability to facilitate drug penetration through the skin barrier. Penetration enhancers such as dimethyl sulfoxide (DMSO), propylene glycol, and various fatty acid derivatives are commonly incorporated to improve drug permeability. These compounds temporarily alter the skin's barrier properties, allowing active ingredients to reach deeper tissue layers where they can exert their therapeutic effects.
Oleic acid and its derivatives serve as effective penetration enhancers by disrupting the lipid organization in the stratum corneum, the outermost layer of skin that typically acts as a barrier to drug absorption. The selection of appropriate enhancers requires consideration of the specific active ingredient properties, target tissue depth, and potential for skin irritation. Advanced formulations may employ multiple enhancers with complementary mechanisms to optimize drug delivery efficiency.
Cyclodextrins represent an innovative class of penetration enhancers that form inclusion complexes with active ingredients, improving their solubility and stability while facilitating controlled release. These cyclic oligosaccharides can significantly enhance the bioavailability of poorly soluble drugs and provide sustained release characteristics that extend the therapeutic duration of the patch application.
Matrix Components and Adhesive Systems
The matrix or base material of transdermal patches plays a crucial role in determining drug release kinetics and overall patch performance. Hydrogel matrices, composed of cross-linked polymers such as polyacrylic acid or polyvinyl alcohol, provide excellent drug loading capacity and controlled release properties. These water-swellable polymers can accommodate both hydrophilic and lipophilic drugs while maintaining flexibility and comfort during wear.
Pressure-sensitive adhesives ensure proper patch adherence to the skin surface throughout the intended wear period while allowing for painless removal. Modern adhesive formulations often incorporate drug-compatible polymers that do not interfere with active ingredient release or stability. The adhesive layer must maintain its properties under various environmental conditions, including exposure to moisture, temperature fluctuations, and physical activity.
Backing layers, typically composed of polyethylene or polyurethane films, provide structural integrity and control the direction of drug release. These impermeable barriers prevent drug loss through the outer surface of the patch while protecting the formulation from environmental contamination. The thickness and flexibility of backing materials are optimized to balance durability with user comfort and discretion.
Specialized Formulation Technologies and Innovations
Microencapsulation and Sustained Release Systems
Advanced patch formulations increasingly utilize microencapsulation technologies to achieve precise control over drug release rates and duration. Microspheres containing active ingredients can be engineered to release their contents at predetermined intervals, providing pulsatile or extended-release profiles that match the natural rhythm of pain perception. This technology is particularly valuable for managing chronic pain conditions that require consistent medication levels over extended periods.
Liposomal delivery systems represent another sophisticated approach to enhancing drug delivery in topical applications. These phospholipid vesicles can encapsulate both water-soluble and lipid-soluble drugs, protecting them from degradation while facilitating deeper skin penetration. The biocompatible nature of liposomes reduces the risk of skin irritation while improving the therapeutic index of incorporated active ingredients.
Nanoparticle formulations enable the incorporation of drugs that would otherwise be unsuitable for topical delivery due to molecular size or stability limitations. These ultra-small carriers can navigate through skin barriers more effectively than conventional formulations, potentially enabling the topical delivery of larger molecules such as peptides or proteins that could revolutionize pain management approaches.
Temperature-Responsive and Smart Delivery Systems
Innovative patch technologies now incorporate temperature-responsive polymers that modify drug release rates based on skin temperature changes. These smart systems can increase medication delivery during periods of increased inflammation when skin temperature typically rises, providing enhanced therapeutic response when needed most. The thermosensitive properties of these materials allow for automatic dose adjustment without user intervention.
Phase-change materials integrated into patch formulations can provide additional therapeutic benefits through temperature modulation. These compounds absorb or release heat during phase transitions, potentially offering cooling or warming effects that complement the pharmacological action of active ingredients. This dual-action approach addresses both the underlying pathophysiology and symptomatic aspects of pain conditions.
pH-responsive delivery systems represent another frontier in smart patch technology, with formulations that can adjust drug release based on local tissue pH changes associated with inflammation or infection. These responsive systems offer the potential for personalized medicine approaches where treatment intensity automatically adjusts to disease severity markers.
Quality Control and Safety Considerations
Manufacturing Standards and Regulatory Compliance
The production of therapeutic patches requires adherence to stringent pharmaceutical manufacturing standards, including Good Manufacturing Practice (GMP) guidelines and specific regulatory requirements for topical drug delivery systems. Quality control protocols must address uniformity of drug content, adhesion properties, release rate consistency, and microbiological safety throughout the product shelf life.
Analytical testing methods for patch products include sophisticated techniques for measuring drug content uniformity, release kinetics under controlled conditions, and adhesive performance under various environmental stresses. These comprehensive testing protocols ensure that each patch delivers the intended dose profile and maintains therapeutic efficacy throughout its designated use period.
Stability studies conducted under accelerated aging conditions provide crucial data about product shelf life and storage requirements. The complex nature of multi-component patch formulations requires extensive compatibility testing to ensure that all ingredients remain stable and effective throughout the product lifecycle. Temperature cycling, humidity exposure, and photostability testing help identify potential degradation pathways and optimize storage recommendations.
Skin Compatibility and Safety Profiles
Dermatological safety represents a paramount concern in patch development, requiring comprehensive evaluation of skin irritation potential, sensitization risk, and compatibility with various skin types. Patch testing protocols typically include both acute and chronic exposure studies to identify potential adverse reactions and establish safe use guidelines for different patient populations.
The selection of inactive ingredients must consider their potential for causing allergic reactions or contact dermatitis, particularly in individuals with sensitive skin or known allergies. Hypoallergenic formulations may exclude common sensitizers such as fragrances, dyes, or preservatives that could trigger adverse reactions in susceptible individuals.
Biocompatibility testing ensures that all patch components are safe for prolonged skin contact and do not cause systemic toxicity through absorption. These studies evaluate both local and systemic effects of patch ingredients, providing comprehensive safety data that supports regulatory approval and clinical use recommendations.
FAQ
What makes hydrogel-based patches different from traditional pain relief patches?
Hydrogel-based patches offer superior comfort and flexibility compared to traditional formulations due to their high water content and polymer matrix structure. These patches conform better to body contours, maintain moisture balance at the application site, and typically provide more consistent drug release rates. The hydrogel matrix also tends to be less irritating to sensitive skin and allows for easier removal without residue or discomfort.
How long should pain relief patches typically be worn for optimal effectiveness?
The optimal wear time varies depending on the specific formulation and active ingredients, but most patches are designed for 8 to 12-hour application periods. Some extended-release formulations may provide therapeutic benefits for up to 24 hours. It is important to follow manufacturer guidelines and healthcare provider recommendations, as exceeding recommended wear times may increase the risk of skin irritation or adverse reactions without providing additional therapeutic benefits.
Can pain relief patches be used in combination with oral pain medications?
While topical patches generally have lower systemic absorption than oral medications, combining treatments should only be done under healthcare provider guidance. Some active ingredients, particularly NSAIDs, may have additive effects when used both topically and orally, potentially increasing the risk of side effects. Healthcare professionals can provide personalized recommendations based on individual medical history, current medications, and specific pain management needs.
Are there any skin conditions that would prevent the use of pain relief patches?
Certain skin conditions may contraindicate patch use or require special precautions. These include active skin infections, open wounds, severe eczema, or psoriasis at the application site. Individuals with known allergies to adhesives, specific active ingredients, or preservatives should carefully review patch ingredients before use. Those with compromised skin barrier function may experience increased systemic absorption and should consult healthcare providers before using topical patches.
Table of Contents
- Active Pharmaceutical Ingredients in Topical Pain Management
- Supporting Components and Delivery Enhancement Systems
- Specialized Formulation Technologies and Innovations
- Quality Control and Safety Considerations
-
FAQ
- What makes hydrogel-based patches different from traditional pain relief patches?
- How long should pain relief patches typically be worn for optimal effectiveness?
- Can pain relief patches be used in combination with oral pain medications?
- Are there any skin conditions that would prevent the use of pain relief patches?